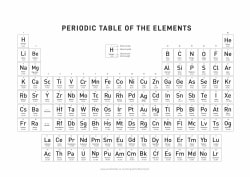
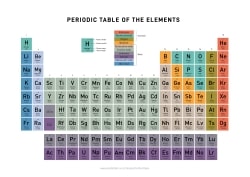
Periodic Table of Elements
|
1
H
Hydrogen
|
2
He
Helium
|
||||||||||||||||
|
3
Li
Lithium
|
4
Be
Beryllium
|
5
B
Boron
|
6
C
Carbon
|
7
N
Nitrogen
|
8
O
Oxygen
|
9
F
Fluorine
|
10
Ne
Neon
|
||||||||||
|
11
Na
Sodium
|
12
Mg
Magnesium
|
13
Al
Aluminum
|
14
Si
Silicon
|
15
P
Phosphorus
|
16
S
Sulfur
|
17
Cl
Chlorine
|
18
Ar
Argon
|
||||||||||
|
19
K
Potassium
|
20
Ca
Calcium
|
21
Sc
Scandium
|
22
Ti
Titanium
|
23
V
Vanadium
|
24
Cr
Chromium
|
25
Mn
Manganese
|
26
Fe
Iron
|
27
Co
Cobalt
|
28
Ni
Nickel
|
29
Cu
Copper
|
30
Zn
Zinc
|
31
Ga
Gallium
|
32
Ge
Germanium
|
33
As
Arsenic
|
34
Se
Selenium
|
35
Br
Bromine
|
36
Kr
Krypton
|
|
37
Rb
Rubidium
|
38
Sr
Strontium
|
39
Y
Yttrium
|
40
Zr
Zirconium
|
41
Nb
Niobium
|
42
Mo
Molybdenum
|
43
Tc
Technetium
|
44
Ru
Ruthenium
|
45
Rh
Rhodium
|
46
Pd
Palladium
|
47
Ag
Silver
|
48
Cd
Cadmium
|
49
In
Indium
|
50
Sn
Tin
|
51
Sb
Antimony
|
52
Te
Tellurium
|
53
I
Iodine
|
54
Xe
Xenon
|
|
55
Cs
Cesium
|
56
Ba
Barium
|
L |
72
Hf
Hafnium
|
73
Ta
Tantalum
|
74
W
Tungsten
|
75
Re
Rhenium
|
76
Os
Osmium
|
77
Ir
Iridium
|
78
Pt
Platinum
|
79
Au
Gold
|
80
Hg
Mercury
|
81
Tl
Thallium
|
82
Pb
Lead
|
83
Bi
Bismuth
|
84
Po
Polonium
|
85
At
Astatine
|
86
Rn
Radon
|
|
87
Fr
Francium
|
88
Ra
Radium
|
A |
104
Rf
Rutherfordium
|
105
Db
Dubnium
|
106
Sg
Seaborgium
|
107
Bh
Bohrium
|
108
Hs
Hassium
|
109
Mt
Meitnerium
|
110
Ds
Darmstadtium
|
111
Rg
Roentgenium
|
112
Cn
Copernicium
|
113
Nh
Nihonium
|
114
Fl
Flerovium
|
115
Mc
Moscovium
|
116
Lv
Livermorium
|
117
Ts
Tennessine
|
118
Og
Oganesson
|
|
57
La
Lanthanum
|
58
Ce
Cerium
|
59
Pr
Praseodymium
|
60
Nd
Neodymium
|
61
Pm
Promethium
|
62
Sm
Samarium
|
63
Eu
Europium
|
64
Gd
Gadolinium
|
65
Tb
Terbium
|
66
Dy
Dysprosium
|
67
Ho
Holmium
|
68
Er
Erbium
|
69
Tm
Thulium
|
70
Yb
Ytterbium
|
71
Lu
Lutetium
|
|||
|
89
Ac
Actinium
|
90
Th
Thorium
|
91
Pa
Protactinium
|
92
U
Uranium
|
93
Np
Neptunium
|
94
Pu
Plutonium
|
95
Am
Americium
|
96
Cm
Curium
|
97
Bk
Berkelium
|
98
Cf
Californium
|
99
Es
Einsteinium
|
100
Fm
Fermium
|
101
Md
Mendelevium
|
102
No
Nobelium
|
103
Lr
Lawrencium
|
Periodic Table of Elements download:
List of all the elements and their properties:
| Atomic Number | Element Name | Symbol | Atomic Weight | Electronegativity |
|---|---|---|---|---|
| 1 | Hydrogen | H | 1.008 | 2.20 |
| 2 | Helium | He | 4.003 | 0.00 |
| 3 | Lithium | Li | 6.941 | 0.98 |
| 4 | Beryllium | Be | 9.012 | 1.57 |
| 5 | Boron | B | 10.811 | 2.04 |
| 6 | Carbon | C | 12.011 | 2.55 |
| 7 | Nitrogen | N | 14.007 | 3.04 |
| 8 | Oxygen | O | 15.999 | 3.44 |
| 9 | Fluorine | F | 18.998 | 3.98 |
| 10 | Neon | Ne | 20.180 | 0.00 |
| 11 | Sodium | Na | 22.990 | 0.93 |
| 12 | Magnesium | Mg | 24.305 | 1.31 |
| 13 | Aluminum | Al | 26.982 | 1.61 |
| 14 | Silicon | Si | 28.086 | 1.90 |
| 15 | Phosphorus | P | 30.974 | 2.19 |
| 16 | Sulfur | S | 32.065 | 2.58 |
| 17 | Chlorine | Cl | 35.453 | 3.16 |
| 18 | Argon | Ar | 39.948 | 0.00 |
| 19 | Potassium | K | 39.098 | 0.82 |
| 20 | Calcium | Ca | 40.078 | 1.00 |
| 21 | Scandium | Sc | 44.956 | 1.36 |
| 22 | Titanium | Ti | 47.867 | 1.54 |
| 23 | Vanadium | V | 50.942 | 1.63 |
| 24 | Chromium | Cr | 51.996 | 1.66 |
| 25 | Manganese | Mn | 54.938 | 1.55 |
| 26 | Iron | Fe | 55.845 | 1.83 |
| 27 | Cobalt | Co | 58.933 | 1.88 |
| 28 | Nickel | Ni | 58.693 | 1.91 |
| 29 | Copper | Cu | 63.546 | 1.90 |
| 30 | Zinc | Zn | 65.390 | 1.65 |
| 31 | Gallium | Ga | 69.723 | 1.81 |
| 32 | Germanium | Ge | 72.640 | 2.01 |
| 33 | Arsenic | As | 74.922 | 2.18 |
| 34 | Selenium | Se | 78.960 | 2.55 |
| 35 | Bromine | Br | 79.904 | 2.96 |
| 36 | Krypton | Kr | 83.800 | 3.00 |
| 37 | Rubidium | Rb | 85.468 | 0.82 |
| 38 | Strontium | Sr | 87.620 | 0.95 |
| 39 | Yttrium | Y | 88.906 | 1.22 |
| 40 | Zirconium | Zr | 91.224 | 1.33 |
| 41 | Niobium | Nb | 92.906 | 1.60 |
| 42 | Molybdenum | Mo | 95.940 | 2.16 |
| 43 | Technetium | Tc | 98.000 | 1.90 |
| 44 | Ruthenium | Ru | 101.070 | 2.20 |
| 45 | Rhodium | Rh | 102.906 | 2.28 |
| 46 | Palladium | Pd | 106.420 | 2.20 |
| 47 | Silver | Ag | 107.868 | 1.93 |
| 48 | Cadmium | Cd | 112.411 | 1.69 |
| 49 | Indium | In | 114.818 | 1.78 |
| 50 | Tin | Sn | 118.710 | 1.96 |
| 51 | Antimony | Sb | 121.760 | 2.05 |
| 52 | Tellurium | Te | 127.600 | 2.10 |
| 53 | Iodine | I | 126.905 | 2.66 |
| 54 | Xenon | Xe | 131.293 | 2.60 |
| 55 | Cesium | Cs | 132.906 | 0.79 |
| 56 | Barium | Ba | 137.327 | 0.89 |
| 57 | Lanthanum | La | 138.906 | 1.10 |
| 58 | Cerium | Ce | 140.116 | 1.12 |
| 59 | Praseodymium | Pr | 140.908 | 1.13 |
| 60 | Neodymium | Nd | 144.240 | 1.14 |
| 61 | Promethium | Pm | 145.000 | 0.00 |
| 62 | Samarium | Sm | 150.360 | 1.17 |
| 63 | Europium | Eu | 151.964 | 0.00 |
| 64 | Gadolinium | Gd | 157.250 | 1.20 |
| 65 | Terbium | Tb | 158.925 | 0.00 |
| 66 | Dysprosium | Dy | 162.500 | 1.22 |
| 67 | Holmium | Ho | 164.930 | 1.23 |
| 68 | Erbium | Er | 167.259 | 1.24 |
| 69 | Thulium | Tm | 168.934 | 1.25 |
| 70 | Ytterbium | Yb | 173.040 | 0.00 |
| 71 | Lutetium | Lu | 174.967 | 1.27 |
| 72 | Hafnium | Hf | 178.490 | 1.30 |
| 73 | Tantalum | Ta | 180.948 | 1.50 |
| 74 | Tungsten | W | 180.948 | 2.36 |
| 75 | Rhenium | Re | 186.207 | 1.90 |
| 76 | Osmium | Os | 190.230 | 2.20 |
| 77 | Iridium | Ir | 192.217 | 2.20 |
| 78 | Platinum | Pt | 195.078 | 2.28 |
| 79 | Gold | Au | 196.967 | 2.54 |
| 80 | Mercury | Hg | 200.590 | 2.00 |
| 81 | Thallium | Tl | 204.383 | 1.62 |
| 82 | Lead | Pb | 207.200 | 2.33 |
| 83 | Bismuth | Bi | 208.980 | 2.02 |
| 84 | Polonium | Po | 209.000 | 2.00 |
| 85 | Astatine | At | 210.000 | 2.20 |
| 86 | Radon | Rn | 222.000 | 0.00 |
| 87 | Francium | Fr | 223.000 | 0.70 |
| 88 | Radium | Ra | 226.000 | 0.90 |
| 89 | Actinium | Ac | 227.000 | 1.10 |
| 90 | Thorium | Th | 232.038 | 1.30 |
| 91 | Protactinium | Pa | 231.036 | 1.50 |
| 92 | Uranium | U | 238.029 | 1.38 |
| 93 | Neptunium | Np | 237.000 | 1.36 |
| 94 | Plutonium | Pu | 244.000 | 1.28 |
| 95 | Americium | Am | 243.000 | 1.30 |
| 96 | Curium | Cm | 247.000 | 1.30 |
| 97 | Berkelium | Bk | 247.000 | 1.30 |
| 98 | Californium | Cf | 251.000 | 1.30 |
| 99 | Einsteinium | Es | 252.000 | 1.30 |
| 100 | Fermium | Fm | 257.000 | 1.30 |
| 101 | Mendelevium | Md | 258.000 | 1.30 |
| 102 | Nobelium | No | 259.000 | 1.30 |
| 103 | Lawrencium | Lr | 262.000 | Unknown |
| 104 | Rutherfordium | Rf | 261.000 | Unknown |
| 105 | Dubnium | Db | 262.000 | Unknown |
| 106 | Seaborgium | Sg | 266.000 | Unknown |
| 107 | Bohrium | Bh | 264.000 | Unknown |
| 108 | Hassium | Hs | 277.000 | Unknown |
| 109 | Meitnerium | Mt | 278.000 | Unknown |
| 110 | Darmstadtium | Ds | 281.000 | Unknown |
| 111 | Roentgenium | Rg | 282.000 | Unknown |
| 112 | Copernicium | Cn | 285.000 | Unknown |
| 113 | Nihonium | Nh | 286.000 | Unknown |
| 114 | Flerovium | Fl | 289.000 | Unknown |
| 115 | Moscovium | Mc | 290.000 | Unknown |
| 116 | Livermorium | Lv | 293.000 | Unknown |
| 117 | Tennessine | Ts | 294.000 | Unknown |
| 118 | Oganesson | Og | 294.000 | Unknown |
You may also like:
Periodic Table+ blog of the month:
Frequently Asked Questions about the Periodic Table of Elements
What is the Periodic Table of Elements?
The Periodic Table of Elements is a scientific chart that organizes all known chemical elements based on their atomic number, electron configuration, and recurring chemical properties. It currently includes 118 confirmed elements, each represented by its symbol, atomic number, and name. The Periodic Table helps chemists, students, and researchers understand relationships between elements and predict chemical reactions.
What is the purpose of the Periodic Table?
The Periodic Table serves as a foundational reference in chemistry and science education. It provides essential information about each element—such as atomic number, symbol, atomic mass, and chemical group. Scientists and educators use the table to identify element properties, classify metals, nonmetals, and metalloids, and predict element behavior in compounds and reactions.
How is the Periodic Table used in real life?
The Periodic Table is widely used in academic, industrial, and research settings. Chemists use it to calculate molar masses, understand bonding tendencies, and balance chemical equations. In education, it helps students grasp the basics of atomic structure and chemical trends. Engineers and manufacturers reference it for selecting materials, especially metals and alloys, based on their reactivity or conductivity.
What do the rows and columns in the Periodic Table mean?
The horizontal rows are called periods, and they indicate increasing atomic numbers from left to right. The vertical columns are called groups or families, and elements in the same group share similar chemical properties. For example, Group 1 elements are alkali metals, while Group 18 contains noble gases.
Who created the Periodic Table of Elements?
The modern Periodic Table was developed by Russian chemist Dmitri Mendeleev in 1869. He arranged the elements by increasing atomic mass and left gaps for undiscovered elements, predicting their properties with remarkable accuracy. Since then, the table has been updated to use atomic number (proton count) as the organizing principle.
Explore our Periodic Table history timeline to learn more about its development.
Why is the Periodic Table important in chemistry?
The Periodic Table is essential in chemistry because it visually summarizes the structure and behavior of elements. It enables quick comparisons of properties like electronegativity, ionization energy, and atomic radius. It also reveals periodic trends that help scientists understand chemical bonding, reaction rates, and compound formation.
Where can I download a free Periodic Table PDF?
You can download high-resolution Periodic Table PDFs in color or black and white formats directly from our website. These printable resources are ideal for classrooms, study sessions, or laboratory use.