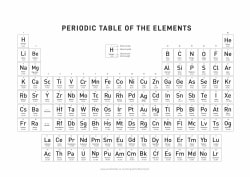
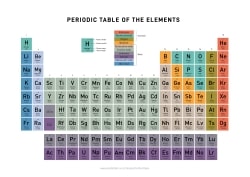
THE PERIODIC TABLE OF ELEMENTS
|
1
H
Hydrogen
|
2
He
Helium
|
||||||||||||||||
|
3
Li
Lithium
|
4
Be
Beryllium
|
5
B
Boron
|
6
C
Carbon
|
7
N
Nitrogen
|
8
O
Oxygen
|
9
F
Fluorine
|
10
Ne
Neon
|
||||||||||
|
11
Na
Sodium
|
12
Mg
Magnesium
|
13
Al
Aluminum
|
14
Si
Silicon
|
15
P
Phosphorus
|
16
S
Sulfur
|
17
Cl
Chlorine
|
18
Ar
Argon
|
||||||||||
|
19
K
Potassium
|
20
Ca
Calcium
|
21
Sc
Scandium
|
22
Ti
Titanium
|
23
V
Vanadium
|
24
Cr
Chromium
|
25
Mn
Manganese
|
26
Fe
Iron
|
27
Co
Cobalt
|
28
Ni
Nickel
|
29
Cu
Copper
|
30
Zn
Zinc
|
31
Ga
Gallium
|
32
Ge
Germanium
|
33
As
Arsenic
|
34
Se
Selenium
|
35
Br
Bromine
|
36
Kr
Krypton
|
|
37
Rb
Rubidium
|
38
Sr
Strontium
|
39
Y
Yttrium
|
40
Zr
Zirconium
|
41
Nb
Niobium
|
42
Mo
Molybdenum
|
43
Tc
Technetium
|
44
Ru
Ruthenium
|
45
Rh
Rhodium
|
46
Pd
Palladium
|
47
Ag
Silver
|
48
Cd
Cadmium
|
49
In
Indium
|
50
Sn
Tin
|
51
Sb
Antimony
|
52
Te
Tellurium
|
53
I
Iodine
|
54
Xe
Xenon
|
|
55
Cs
Cesium
|
56
Ba
Barium
|
L |
72
Hf
Hafnium
|
73
Ta
Tantalum
|
74
W
Tungsten
|
75
Re
Rhenium
|
76
Os
Osmium
|
77
Ir
Iridium
|
78
Pt
Platinum
|
79
Au
Gold
|
80
Hg
Mercury
|
81
Tl
Thallium
|
82
Pb
Lead
|
83
Bi
Bismuth
|
84
Po
Polonium
|
85
At
Astatine
|
86
Rn
Radon
|
|
87
Fr
Francium
|
88
Ra
Radium
|
A |
104
Rf
Rutherfordium
|
105
Db
Dubnium
|
106
Sg
Seaborgium
|
107
Bh
Bohrium
|
108
Hs
Hassium
|
109
Mt
Meitnerium
|
110
Ds
Darmstadtium
|
111
Rg
Roentgenium
|
112
Cn
Copernicium
|
113
Nh
Nihonium
|
114
Fl
Flerovium
|
115
Mc
Moscovium
|
116
Lv
Livermorium
|
117
Ts
Tennessine
|
118
Og
Oganesson
|
|
57
La
Lanthanum
|
58
Ce
Cerium
|
59
Pr
Praseodymium
|
60
Nd
Neodymium
|
61
Pm
Promethium
|
62
Sm
Samarium
|
63
Eu
Europium
|
64
Gd
Gadolinium
|
65
Tb
Terbium
|
66
Dy
Dysprosium
|
67
Ho
Holmium
|
68
Er
Erbium
|
69
Tm
Thulium
|
70
Yb
Ytterbium
|
71
Lu
Lutetium
|
|||
|
89
Ac
Actinium
|
90
Th
Thorium
|
91
Pa
Protactinium
|
92
U
Uranium
|
93
Np
Neptunium
|
94
Pu
Plutonium
|
95
Am
Americium
|
96
Cm
Curium
|
97
Bk
Berkelium
|
98
Cf
Californium
|
99
Es
Einsteinium
|
100
Fm
Fermium
|
101
Md
Mendelevium
|
102
No
Nobelium
|
103
Lr
Lawrencium
|
Download the Periodic Table of Elements in PDF format:
Blog of the month:
List of all the elements and their properties:
| Atomic Number | Element Name | Symbol | Atomic Weight | Electronegativity |
|---|---|---|---|---|
| 1 | Hydrogen | H | 1.008 | 2.20 |
| 2 | Helium | He | 4.003 | 0.00 |
| 3 | Lithium | Li | 6.941 | 0.98 |
| 4 | Beryllium | Be | 9.012 | 1.57 |
| 5 | Boron | B | 10.811 | 2.04 |
| 6 | Carbon | C | 12.011 | 2.55 |
| 7 | Nitrogen | N | 14.007 | 3.04 |
| 8 | Oxygen | O | 15.999 | 3.44 |
| 9 | Fluorine | F | 18.998 | 3.98 |
| 10 | Neon | Ne | 20.180 | 0.00 |
| 11 | Sodium | Na | 22.990 | 0.93 |
| 12 | Magnesium | Mg | 24.305 | 1.31 |
| 13 | Aluminum | Al | 26.982 | 1.61 |
| 14 | Silicon | Si | 28.086 | 1.90 |
| 15 | Phosphorus | P | 30.974 | 2.19 |
| 16 | Sulfur | S | 32.065 | 2.58 |
| 17 | Chlorine | Cl | 35.453 | 3.16 |
| 18 | Argon | Ar | 39.948 | 0.00 |
| 19 | Potassium | K | 39.098 | 0.82 |
| 20 | Calcium | Ca | 40.078 | 1.00 |
| 21 | Scandium | Sc | 44.956 | 1.36 |
| 22 | Titanium | Ti | 47.867 | 1.54 |
| 23 | Vanadium | V | 50.942 | 1.63 |
| 24 | Chromium | Cr | 51.996 | 1.66 |
| 25 | Manganese | Mn | 54.938 | 1.55 |
| 26 | Iron | Fe | 55.845 | 1.83 |
| 27 | Cobalt | Co | 58.933 | 1.88 |
| 28 | Nickel | Ni | 58.693 | 1.91 |
| 29 | Copper | Cu | 63.546 | 1.90 |
| 30 | Zinc | Zn | 65.390 | 1.65 |
| 31 | Gallium | Ga | 69.723 | 1.81 |
| 32 | Germanium | Ge | 72.640 | 2.01 |
| 33 | Arsenic | As | 74.922 | 2.18 |
| 34 | Selenium | Se | 78.960 | 2.55 |
| 35 | Bromine | Br | 79.904 | 2.96 |
| 36 | Krypton | Kr | 83.800 | 3.00 |
| 37 | Rubidium | Rb | 85.468 | 0.82 |
| 38 | Strontium | Sr | 87.620 | 0.95 |
| 39 | Yttrium | Y | 88.906 | 1.22 |
| 40 | Zirconium | Zr | 91.224 | 1.33 |
| 41 | Niobium | Nb | 92.906 | 1.60 |
| 42 | Molybdenum | Mo | 95.940 | 2.16 |
| 43 | Technetium | Tc | 98.000 | 1.90 |
| 44 | Ruthenium | Ru | 101.070 | 2.20 |
| 45 | Rhodium | Rh | 102.906 | 2.28 |
| 46 | Palladium | Pd | 106.420 | 2.20 |
| 47 | Silver | Ag | 107.868 | 1.93 |
| 48 | Cadmium | Cd | 112.411 | 1.69 |
| 49 | Indium | In | 114.818 | 1.78 |
| 50 | Tin | Sn | 118.710 | 1.96 |
| 51 | Antimony | Sb | 121.760 | 2.05 |
| 52 | Tellurium | Te | 127.600 | 2.10 |
| 53 | Iodine | I | 126.905 | 2.66 |
| 54 | Xenon | Xe | 131.293 | 2.60 |
| 55 | Cesium | Cs | 132.906 | 0.79 |
| 56 | Barium | Ba | 137.327 | 0.89 |
| 57 | Lanthanum | La | 138.906 | 1.10 |
| 58 | Cerium | Ce | 140.116 | 1.12 |
| 59 | Praseodymium | Pr | 140.908 | 1.13 |
| 60 | Neodymium | Nd | 144.240 | 1.14 |
| 61 | Promethium | Pm | 145.000 | 0.00 |
| 62 | Samarium | Sm | 150.360 | 1.17 |
| 63 | Europium | Eu | 151.964 | 0.00 |
| 64 | Gadolinium | Gd | 157.250 | 1.20 |
| 65 | Terbium | Tb | 158.925 | 0.00 |
| 66 | Dysprosium | Dy | 162.500 | 1.22 |
| 67 | Holmium | Ho | 164.930 | 1.23 |
| 68 | Erbium | Er | 167.259 | 1.24 |
| 69 | Thulium | Tm | 168.934 | 1.25 |
| 70 | Ytterbium | Yb | 173.040 | 0.00 |
| 71 | Lutetium | Lu | 174.967 | 1.27 |
| 72 | Hafnium | Hf | 178.490 | 1.30 |
| 73 | Tantalum | Ta | 180.948 | 1.50 |
| 74 | Tungsten | W | 180.948 | 2.36 |
| 75 | Rhenium | Re | 186.207 | 1.90 |
| 76 | Osmium | Os | 190.230 | 2.20 |
| 77 | Iridium | Ir | 192.217 | 2.20 |
| 78 | Platinum | Pt | 195.078 | 2.28 |
| 79 | Gold | Au | 196.967 | 2.54 |
| 80 | Mercury | Hg | 200.590 | 2.00 |
| 81 | Thallium | Tl | 204.383 | 1.62 |
| 82 | Lead | Pb | 207.200 | 2.33 |
| 83 | Bismuth | Bi | 208.980 | 2.02 |
| 84 | Polonium | Po | 209.000 | 2.00 |
| 85 | Astatine | At | 210.000 | 2.20 |
| 86 | Radon | Rn | 222.000 | 0.00 |
| 87 | Francium | Fr | 223.000 | 0.70 |
| 88 | Radium | Ra | 226.000 | 0.90 |
| 89 | Actinium | Ac | 227.000 | 1.10 |
| 90 | Thorium | Th | 232.038 | 1.30 |
| 91 | Protactinium | Pa | 231.036 | 1.50 |
| 92 | Uranium | U | 238.029 | 1.38 |
| 93 | Neptunium | Np | 237.000 | 1.36 |
| 94 | Plutonium | Pu | 244.000 | 1.28 |
| 95 | Americium | Am | 243.000 | 1.30 |
| 96 | Curium | Cm | 247.000 | 1.30 |
| 97 | Berkelium | Bk | 247.000 | 1.30 |
| 98 | Californium | Cf | 251.000 | 1.30 |
| 99 | Einsteinium | Es | 252.000 | 1.30 |
| 100 | Fermium | Fm | 257.000 | 1.30 |
| 101 | Mendelevium | Md | 258.000 | 1.30 |
| 102 | Nobelium | No | 259.000 | 1.30 |
| 103 | Lawrencium | Lr | 262.000 | Unknown |
| 104 | Rutherfordium | Rf | 261.000 | Unknown |
| 105 | Dubnium | Db | 262.000 | Unknown |
| 106 | Seaborgium | Sg | 266.000 | Unknown |
| 107 | Bohrium | Bh | 264.000 | Unknown |
| 108 | Hassium | Hs | 277.000 | Unknown |
| 109 | Meitnerium | Mt | 278.000 | Unknown |
| 110 | Darmstadtium | Ds | 281.000 | Unknown |
| 111 | Roentgenium | Rg | 282.000 | Unknown |
| 112 | Copernicium | Cn | 285.000 | Unknown |
| 113 | Nihonium | Nh | 286.000 | Unknown |
| 114 | Flerovium | Fl | 289.000 | Unknown |
| 115 | Moscovium | Mc | 290.000 | Unknown |
| 116 | Livermorium | Lv | 293.000 | Unknown |
| 117 | Tennessine | Ts | 294.000 | Unknown |
| 118 | Oganesson | Og | 294.000 | Unknown |
Frequently asked questions:
What is the Periodic Table of Elements?
The Periodic table of elements is a tool, developed by scientists over hundreds of years. The table lists all the elements that are currently known (118), in descending order of the number of protons that are present, in a single atom of the element.
How is the Periodic Table of Elements used?
The Periodic Table of Elements can be used as an assisting tool in chemical calculations, when a specification of an element is needed it is easily found in the Periodic Table. The periodic table also gives us an idea of what the characteristics of an element might be and help us predict how an element might react based on in which group it is located.
Who invented the Periodic Table of Elements?
The Periodic Table has constantly been improved and developed over the past 200 years, but in 1869 Dimitri Mendeleev finished the first version of the periodic table as we know it today, by arranging the elements by atomic mass and leaving spaces open for the elements that were not yet discovered.
We created a timeline of the history of the periodic table..